Background: Indolent B-cell lymphoma (iBCL) are clinically heterogeneous and accounts for 10-15% of all kinds of subtypes in NHL in China. Although iBCL have a relatively good survival, majority of these lymphomas is considered incurable. The favorable activity and safety profile of rituximab monotherapy in the initial treatment of iBCL has been proven. Interferon alpha (IFN-α) was effective in modulating immune responses and may enhance the clinical efficacy of rituximab in vitro and in vivo studies. Clinical combination of rituximab and conventional IFN-α was associated with fewer early treatment failures compared to single agent rituximab. Peginterferon-α-2(peg-IFN-α2) has a longer half-life with less toxicity. It is also recommended as one of the therapeutic options for chronic hepatitis B virus (CHB) infection. The RIPPLE study (ClinicalTrials. gov identifier: NCT04246359) was initiated to evaluate the efficacy and safety of rituximab plus pegylated interferon α-2b for treatment-naive patients with iBCL. This publication provides long-term follow-up data.
Methods: This trial enrolled pts aged 18-80 years with newly diagnosed iBCL including follicular lymphoma (FL, grade 1-2,3a), marginal zone lymphoma (MZL), lymphoplasmacytic lymphoma (LPL), small lymphocytic lymphoma (SLL). Pts were eligible if they had ECOG ≤ 2, adequate organ function and bone marrow function, and at least one measurable or evaluable lesion. Further eligibility criteria were HBsAg positivity with HBV DNA load <3000 IU/mL prior to study; serum ALT level of <5 times the upper limit of normal. During induction phase, pts received rituximab biosimilar (Henliritux ® Shanghai Henlius Biotech) 375 mg/m 2 intravenous infusion on d1. Peg-IFN-α2b (Pegberon ®, Xiamen Amoytop Biotech) was given at a dose of 135ug, subcutaneously, on d1,8. The combination repeated every 21 days for 6 cycles. Responded pts receive rituximab (every 2 months) plus peg-IFN-α2b (every month) maintenance at the dose described above, for up to 2 years until disease progression and intolerance. Simultaneously, CHB pts orally treated with entecavir continuously. The primary endpoint is ORR assessed by investigators per Lugano 2014 criteria. Key secondary endpoints included TTR, DOR, PFS, OS, HBV DNA load clearance, and safety. Adverse events (AEs) were summarized according to NCI CTCAE v5.0.
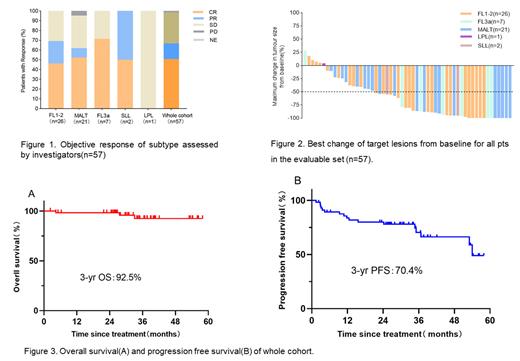
Results: From September 2018 to July 2021, 57 eligible pts with median age of 54 (range, 29-75) years were enrolled from 6 institutions in China. Thirty (56.1%) are female, 21(36.8%) pts were symptomatic and 22(38.6%) pts with FLIPI score≥3, 6(10.5%) pts suffer from hepatitis B with HBV DNA load <3000 IU/mL at study entry. At cutoff date, there were 26(45.6%) FL (grade 1-2), 21(36.8%) MALT, 7(12.3%) FL (grade 3a) , 2(3.5%) SLL, 1(1.8) LPL pts enrolled. Of 57 response evaluable pts, 38(66.7%) pts achieved an objective response including 29(50.9%) pts with CR based on investigators. ORR were 69.2% (18/26), 71.4% (5/7), 61.9% (13/21), 0, 100% (2/2) and the CR rates were 46.2% (12/26), 71.4% (5/7), 52.4% (11/21), 0, 50% (1/2) for FL (grade 1-2), FL (grade3a), MALT, LPL, SLL, respectively (Figure 1-2). Median HBV DNA load clearance time was 1.8 months (1.3-2.1months), no hepatitis B virus reactivation reported. With median follow-up time was 34.2 months (IQR:26.5-42.3 months), median OS, PFS and DoR was not reached; 3-years OS and PFS rate of whole cohort was 92.5%, 70.4%, respectively (Figure 3 C-D). Median TTR was 3.0months (1.5-5.2months). The most common treatment-emergent adverse events (TEAEs) were hematological relevance toxicities. The most common hematological TEAEs (>30%) were neutropenia 63.2% (36/57), anemia 36.5% (19/57), thrombocytopenia 29.8% (17/57). Non-hematological TEAEs (>10%) were fever (28.1%, 16/57), transaminase elevated (26.3%, 15/57), fatigue (26.3%, 15/57), infusion reaction (21.7%, 10/46). No treatment-related death occurred.
Conclusion: Rituximab biosimilar plus pegylated interferon α-2b provided favorable response in newly diagnosed advanced iBCL with mild toxicity. No hepatitis B virus reactivation was observed.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal